On November 22, Nature Computational Science published the latest research results on molecular simulation of supercapacitors in an article entitled "Modeling galvanostatic charge-discharge of nanoporous supercapacitors". The results are achieved by the Prof. Feng Guang's team of the State Key Laboratory of Coal Combustion (SKLCC) of HUST School of Energy and Power Engineering (EPE), with Huazhong University of Science and Technology (HUST) as the first unit, the team's Dr. Zeng Liang and postgraduate Wu Taizheng as the first and second authors, respectively, and Prof. Feng Guang as the corresponding author.
Advanced energy storage technology is indispensable in achieving China's "emission peak and carbon neutrality" goals. Given their advantages such as high power density, long cycle life, and wide range of operating temperatures, supercapacitors have played an important role in energy storage. As molecular simulation can directly analyze the microstructure and formation process of double electric layers, it is often used to study the energy storage performance and thermodynamic/kinetic energy storage mechanism of supercapacitors, especially supercapacitors with nanoporous electrodes. In the molecular simulation of supercapacitors, how to simulate the polarization of electrodes is the key as compared with related simulation methods. At present, the most widely used method is to evenly distribute the charge on the electrode atoms (that is, the constant charge method). Compared with the constant charge method, the constant potential method is superior in accurately simulating electrode polarization though with a higher computational cost: it adjusts the charge on the electrode atoms according to the applied voltage and the electrolyte environment, so that the electrode stays equipotential during charging and discharging. In the study of the capacity and performance of supercapacitors, the equal charge method can be applied to study an ideal open-loop electrode system, while the simulation of the nanoporous electrode system tends not to be accurate. For the simulation research of supercapacitor charging and discharging and other dynamic processes, only the constant potential method can be used, because this is the only method that can be applied to realize automatic adjustment of electrode (for which the constant charge method is not available), so as to get the correct dynamic process of charging and discharging and accurate quality of heat produced. For the study of the charging and discharging processes of supercapacitors, previous molecular simulations were either based on the constant charge method or in adoption of voltage regulation mode, and could not explore the energy storage mechanism under current control mode. Considering that the current mode has been widely used in actual measurement of supercapacitors and basic electrochemical researches (such as Galvanostatic Charge/Discharge (GCD)), it is in urgent need of a molecular simulation method that could realize current regulation while keeping the electrode potential constant, to accurately simulate the charging and discharging process of supercapacitors under current regulation.
In response to the problems mentioned above, the team led by Prof. Feng Guang developed a molecular simulation method based on the constant potential method to simulate the GCD process of supercapacitors. The results show that for supercapacitors with nanoporous electrodes, the simulation method can obtain a charge-discharge process consistent with experimental measurement. At the same time, the method serves for revealing the kinetic hysteresis mechanism of ion electrolyte adsorption-desorption during the GCD process on a molecular scale, which is a result of the lagging of electrolyte response behind the electrode and charge response to polarization.
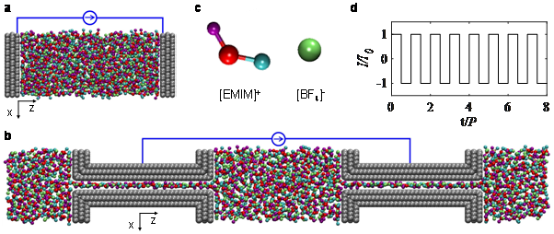
This newly proposed method can not only simulate the charging and discharging process of supercapacitors under any form of current, but also can be combined with the constant potential method of voltage regulation mode to comprehend the dynamics of the charging and discharging process and obtain the optimal charging and discharging mode.. Meanwhile, it can also be applied to simulate other dynamic formation processes involving double electrode layers, such as batteries, capacitive deionization (CDI), electrowetting, and electrolyte gating. In addition, this work is also of guiding significance to the choice of molecular simulation methods for different simulation systems.
This work wassupported by the National Natural Science Foundation of China, the Natural Science Foundation of Hubei Province, and the HUST Academic Frontier Youth Team Program.
Prof. Feng Guang has been engaged in basic researches on energy storage mechanism and optimization design of supercapacitors since he worked in EPE in 2013. Featuring interdisciplinary field, his research directions include energy, physics, chemistry, and materials, etc., in which he has made some innovative achievements over the past three years (these innovative results have been published in some top journals like Nature Materials, Nature Computational Science, Nature Communications, and Physical Review X with HUST as the first unit).
Paper Link: https://www.nature.com/articles/s43588-021-00153-5




